Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Pre-Clinical Evaluation of Left Atrial Appendage Closure Device in Mitigating Risk of Stroke in Patients with Atrial Fibrillation: Promising Results from Animal Trials with Future Prospects
Authors: Dr. Pramodkumar Minocha, Dr. Deveshkumar Mahendralal Kothwala, Arpit Pradipkumar Dave, Mahesh Laxman Pawar, Hrishikesh Mahendra Shah , Sanjay Baijnath Chauhan
DOI Link: https://doi.org/10.22214/ijraset.2024.59277
Certificate: View Certificate
Abstract
Atrial fibrillation (AF) is the most common clinical disorder of cardiac arrhythmia in geriatrics, characterized by the presence of disorganized and irregular electrical activity in atrial myocardium, which leads to worsening of the heart\'s systolic function. Left atrial appendage (LAA) is a tubular, tiny aperture construction, connecting the left atrium (LA) with separate structure from LA with distinctive mechanical, hormonal, and structural properties. AF and Mitral valve regurgitation disease elevate the chances of thrombus formation in LAA, these ultimately turns into stroke. Obstructing thrombus by using a Left Atrial Appendage Closure Device (LAAC) and preventing it from entering internal carotid arteries, the risk of LAA thrombosis can be decreased. This article serves as a comprehensive compilation detailing the successful development and implantation of Left Atrial Appendage Closure (LAAC) Device with its novel design and geometry in two pigs (pre-clinical evaluation), showcasing promising results that have future implications for addressing cardiovascular disease in humans.
Introduction
I. INTRODUCTION
Stroke is a condition in which carotid arteries get block and blood flow get reduce towards brain, at some point it will cause paralysis. There are multiple reasons for stroke for example, high blood pressure, diabetes, smoking or atrial fibrillation and mitral valve like heart disease. Patients with atrial fibrillation (AF) have a three to five fold elevated risk for the occurrence of stroke compared with individuals without AF, because of irregular heartbeat. The majority cases of AF stroke, formation of clots or thrombus is due to left atrial appendage (LAA). In individuals with nonvalvular AF, the left atrial appendage (LAA), a blind pouch of the left atrium (LA), is responsible for around 90% of cardiac thrombi.
The left atrial appendage (LAA), which is distinct from the left atrium proper in terms of development, ultrastructure and physiology, develops from the left wall of the main atrium during the fourth week of embryonic development. The most accurate non-invasive imaging modalities for defining LAA anatomy and topographic relationships are transesophageal echocardiograms (TEE) and multi-detector CT with high definition. With the advent of three-dimensional reconstructions, TEE has become even more precise. Its inferolateral section is tightly associated with the pericardium and free wall of the left ventricle. A traditional echocardiogram is done by putting the transducer on the surface of the chest. This is called a transthoracic echocardiogram. A transesophageal echocardiogram is done by inserting a probe with a transducer down the esophagus. Because of its anatomical placement, the LAA is typically difficult to see on transthoracic echocardiography (TTE).
In addition to being an embryologic remnant, LAA appears to be crucial for controlling fluid balance and heart rhythm. On the other hand, LAA may cause atrial tachyarrhythmia and plays a significant role in the thromboembolic risk linked to atrial fibrillation (AF). The vital component that LAA serves in thromboembolic events during AF is evident; however there are several cardiovascular surgery approaches that can help lessen the risk, notably surgical LAA obliteration and anticoagulant treatment. Self-expanding nitinol alloy implant produces promising results by retaining thrombus and preventing the entry of LAA clots into the carotid arteries. The current reference standard for diagnosing LAA thrombi is transesophageal echocardiography (TEE).
In summary, this study presents the development and evaluation of the LAAC device, implemented and studied in pigs through a 14Fr delivery sheath. Animal P1 and Animal P2 underwent thorough pre-clinical examinations and surgical preparations, with continuous monitoring of vital signs using electrocardiograms (ECG). The pigs were observed for 30 and 90 days, respectively, housed in appropriate cages and observed for any physical or clinical changes. At the termination of the experiment, necropsy and histopathological evaluations were conducted. The findings suggest that the LAA closure device with its novel design and geometry has serve as a valuable preventive measure against ischemic stroke, providing a safer alternative by effectively trapping blood clots in the Left Atrial Appendage. The study aims to introduce and assess the LAAC device for individuals at high risk of stroke due to cardiovascular diseases such as atrial fibrillation and mitral valve regurgitation. Utilizing this device may prove contributory in preventing thrombus entry into carotid arteries, marking a significant advancement in stroke prevention strategies.
II. MATERIALS AND METHODS
A. Animal Preparation
Two male pigs were chosen for implantation study because pigs have circulatory system needed for comparable to adult human, and also they have characteristics with the desired patient group for the device- such as size, cardiac output, heart rate, blood pressure, etc. Before the surgical procedure, the animals were fasted for at least 12 hours and had no access to water and tag according to their time of observation period, animal P1 for 30 days and animal P2 for 90 days. The animals were weighed, sedated, placed with monitoring equipment, and given a pre-anesthetic dosage of 0.05 mg/kg (IM) of atropine, 15 mg/kg (IM) of ketamine, and 2.5 mg/kg (IM) of xylazine. A facemask was then used to administer a 1-3% inhalation anesthetic. For the application of ECG leads and the aortic root approach, hairs in the groin region were removed.
B. Experimental Procedure
The animals underwent a three-day anticoagulant therapy regimen leading up to the procedure day, and this continued from the implantation of the test item on day 0 (the day of implantation) until the termination of the study. The animals were maintained on a reduced aspirin dose of 150 mg per animal. ACT measurements were conducted both before and after heparinization, which was implemented to maintain ACT values between 250 to 550 seconds.
The percutaneous approach was employed using the Seldinger method, wherein an 18G puncture needle was used to puncture the vessel. An 8F sheath was inserted into the femoral vein, and a 6F sheath was inserted into the femoral artery. Throughout the procedure, continuous monitoring of electrocardiogram (ECG), respiration rate, heart rate, and oxygen saturation was performed and documented in the surgical record.
C. Medications
|
Purpose |
Drug Name |
Conc. |
Dose |
Route |
Frequency |
|
Anti-coagulation |
Aspirin |
NA |
150 mg/Animal |
PO |
Once Daily from Day 0 to till terminal day |
|
Clopidogrel |
NA |
75 mg/Animal |
PO |
Once Daily from Day 0 to till terminal day |
|
|
Heparin |
5000 IU/mL |
100 IU/kg |
IV |
Day 0, before the device implantation (every hour on discretion of interventionist) |
|
|
Pre-anaesthetic |
Atropine |
0.6 mg/mL |
0.05 mg/kg |
IM |
Once before induction anaesthesia |
|
Induction of Anesthesia |
Ketamine |
50 mg/mL |
15 mg/Kg |
IM |
Once to sedate animal |
|
Xylazine |
23.32 mg/mL |
2.5 mg/kg |
IM |
Once to sedate animal |
|
|
Isoflurane |
NA |
1% |
face mask |
Induction |
|
|
Maintenance of Anesthesia |
Isoflurane |
NA |
2.5% |
Inhalation via endo-tracheal tube |
For maintenance |
|
Analgesic |
Tramadol |
50 mg/mL |
2to4 mg/kg |
IM |
Once before procedure |
|
Antibiotic |
Enrofloxacin |
100 mg/mL |
5- 7.5 mg/kg |
IM |
Once before procedure |
|
Euthanasia |
Thiopental sodium |
300 mg/mL |
100 mg/Kg |
IV |
Once at the termination of procedure |
Table 01: "Pharmaceutical Agents Administered to pigs for Preoperative, Intraoperative, and Postoperative Care"
D. Necropsy
Gross necropsy and photography were conducted for the organ where the test item was applied, as well as the downstream organs to assess local effects such as thrombus formation and inflammations. At the scheduled sacrifice dates (P1 on day 30 and P2 on Day 90), all animals were euthanized using an overdose of Thiopental sodium. A pathologist examined the animals for external and internal gross pathological changes. The implanted device was collected and preserved in 10% neutral buffered formalin as per the study protocol. The left atrial appendage and implanted device were prepared for resin embedding and sectioned to approximately 200 microns thickness, followed by thickness reduction through polishing. The investigating pathologist stained the specimens with Hematoxylin & Eosin (H & E) and examined them under a light microscope to evaluate histopathological abnormalities.
III. RESULTS AND DISCUSSION
A. Radiography Analysis
Radiographic imaging was conducted both during and after the implantation to ensure the accuracy of the left atrial appendage closure (LAAC) device position. The post-implant position of LAAC in animal P1 is shown in Figure 01 (A) & (B) respectively at 0 and 30 days. Similarly figure 02 (A) & (B) represents LAAC in animal P2 at 0 and 90 days respectively.

3) Body Weight
No reduction in body weight was observed in the P1 animal, with a starting weight of 32.3 kg on Day 0 and a weight of 35.5 kg on Day 30. Similarly, the P2 animal, starting at 34.3 kg on Day 0, showed no decrease in body weight, reaching 38.2 kg on Day 90.
B. Clinical Pathology Analysis
Hematology and clinical chemistry tests were conducted on blood samples before the procedure and at the terminal stage. The corresponding parameters for terminal haematology and clinical chemistry to the baseline values are shown in the tables below (Tables 2, 3, 4, and 5).
|
Animal No |
P1/Baseline |
P2/Baseline |
SD |
Average |
|
AST (U/L) |
31 |
26 |
3.54 |
20.18 |
|
Ca (mmol/L) |
2.25 |
1.88 |
0.26 |
1.46 |
|
CK (U/L) |
809 |
661 |
104.65 |
524.88 |
|
Create (µmol/L) |
57 |
43 |
9.90 |
36.63 |
|
LDH (U/L) |
483 |
395 |
62.23 |
313.41 |
|
BUN (mmol/L) |
1.99 |
1.68 |
0.22 |
1.30 |
|
Sodium (mmol/L) |
136.4 |
138.8 |
1.70 |
92.30 |
|
Potassium (mmol/L) |
2.98 |
2.37 |
0.43 |
1.93 |
|
Chloride (mmol/L) |
92.5 |
96.3 |
2.69 |
63.83 |
Table 02: "Biochemistry Baseline Parameters (P1 and P2) with Standard Deviation (SD) and Average"
|
Animal No |
P1/Terminal |
P2/Terminal |
|
AST (U/L) |
58 |
45 |
|
Ca (mmol/L) |
2.81 |
3.04 |
|
CK (U/L) |
996 |
1431 |
|
Create (µmol/L) |
63 |
61 |
|
LDH (U/L) |
736 |
417 |
|
BUN (mmol/L) |
3.73 |
4.16 |
|
Sodium (mmol/L) |
141.7 |
138.2 |
|
Potassium (mmol/L) |
4.13 |
3.43 |
|
Chloride (mmol/L) |
110.7 |
98.9 |
Table 03: "Terminal Parameters for P1 and P2"
|
Animal No |
P1/Baseline |
P2/Baseline |
SD |
Average |
|
WBC (109/L) |
15.38 |
13.39 |
1.41 |
10.06 |
|
RBC (1012/L) |
5.45 |
4.81 |
0.45 |
3.57 |
|
HGB (g/L) |
101 |
87 |
9.90 |
65.97 |
|
HCT (L/L) |
0.300 |
0.266 |
0.02 |
0.20 |
|
MCV (fL) |
55.1 |
55.2 |
0.07 |
36.79 |
|
MCH (pg) |
18.5 |
18.0 |
0.35 |
12.28 |
|
MCHC (g/L) |
337 |
327 |
7.07 |
223.69 |
|
PLT (109/L) |
523 |
451 |
50.91 |
341.64 |
|
NEUT (109/L) |
57.3 |
57.7 |
0.28 |
38.43 |
|
LYMPHO (109/L) |
39.5 |
40.0 |
0.35 |
26.62 |
|
MONO (109/L) |
1.6 |
1.2 |
0.28 |
1.03 |
|
EOS (109/L) |
0.2 |
0.1 |
0.07 |
0.12 |
|
BASO (109/L) |
1.4 |
0.9 |
0.35 |
0.88 |
|
LUC (%) |
0.0 |
0.0 |
0.00 |
0.00 |
|
RETIC (%) |
0.75 |
0.62 |
0.09 |
0.49 |
Table 04: "Baseline Hematological Parameters with Standard Deviation (SD) and Average"
|
Animal No |
P1/Terminal |
P2/Terminal |
|
WBC (109/L) |
24.21 |
13.04 |
|
RBC (1012/L) |
4.52 |
5.01 |
|
HGB (g/L) |
91 |
112 |
|
HCT (L/L) |
0.282 |
0.330 |
|
MCV (fL) |
62.3 |
65.8 |
|
MCH (pg) |
20.2 |
22.4 |
|
MCHC (g/L) |
324 |
340 |
|
PLT (109/L) |
223 |
267 |
|
NEUT (109/L) |
9.3 |
38.3 |
|
LYMPHO (109/L) |
76.0 |
58.0 |
|
MONO (109/L) |
13.5 |
1.9 |
|
EOS (109/L) |
0.2 |
0.2 |
|
BASO (109/L) |
0.9 |
1.4 |
|
LUC (%) |
0.1 |
0.1 |
|
RETIC (%) |
0.72 |
0.95 |
Table 05: "Terminal Hematological Parameters for P1 and P2"
C. Necropsy Analysis
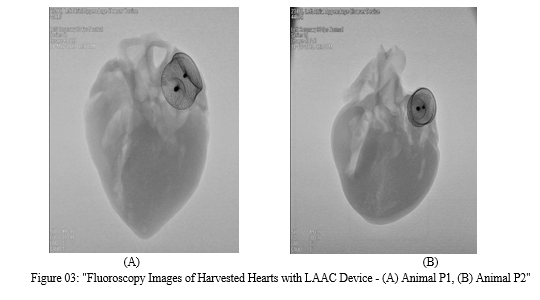
The organs underwent a comprehensive gross evaluation, focusing particularly on identifying evidence of thromboembolism or any other abnormalities by the Hematoxylin & Eosin (H & E) strain method. No abnormal gross findings were detected in any of the examined tissues which is visible in figure 03.
D. Histopathological Analysis
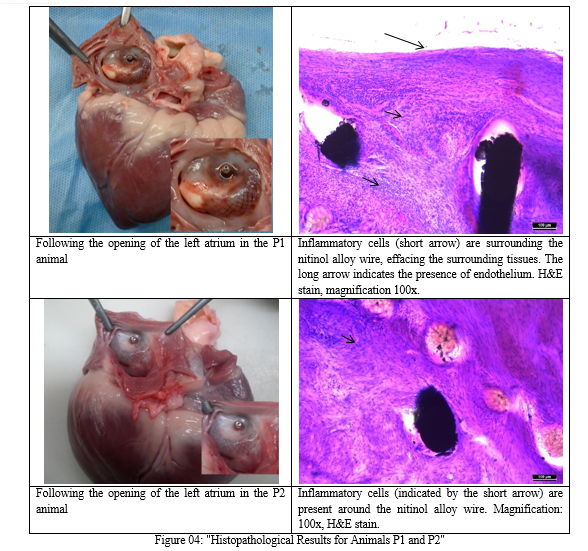
The objective of the histopathological study was to evaluate device ingrowth and biocompatibility through microscopic examination. All devices exhibited partial or complete overgrowth with tissue, dependent on the duration of follow-up (i.e., 30 and 90 days).
The connective tissue was well confined to the device and distinguishable from myocardial tissue. In P1 animals on Day 30, the connective tissue surrounding the nitinol alloy wire showed moderate infiltration of inflammatory cells, effacing the adjacent tissue. On Day 90 in P2 animals, inflammatory cell infiltration was limited to the struts. The presence of follicle-like lymphocytic structures indicated chronic inflammation, observed at both 30 and 90 days post-implantation. The composition of this infiltration decreased from day 30 to day 90.
As shown in figure 04, considering the limited degree and extent of this inflammation, no clinical symptoms are anticipated. Such inflammatory and tissue responses are expected in the context of large device implantation in the endocardium, and these changes were not classified as adverse reactions. In summary, the histopathological examination results suggest good biocompatibility of the device at both 30 and 90 days of follow-up.
- Animal P1
Microscopic examination revealed a substantial presence of inflammatory cells, exposing the surrounding tissue and an endocardial breach. There was no observed loss of smooth muscle cells or deposition of fibrin. The left atrial appendage closure device (LAAC) demonstrated endothelialisation, with over 75% of its surface covered by epithelium, resulting in a total histology score of 4.
2. Animal P2
Microscopic examination illustrates the formation of inflammatory cells around the strut. The overall Histopathology score was 3, indicating a broken endocardium without any loss of smooth muscle cells or fibrin deposition. The entire surface (100%) of the left atrial appendage closure device (LAAC) was covered with epithelium, demonstrating endothelialisation.
Conclusion
In conclusion, the findings from this research present a comprehensive evaluation of the self-expanding nitinol Left Atrial Appendage Closure (LAAC) device in the context of reducing the risk of stroke in atrial fibrillation (AF) patients. The study focused on assessing the device\'s efficacy in pigs during animal trials, employing a variety of criteria such as size, heart rate, blood pressure, cardiac output, and compatibility with adult humans. The selection of two pigs, labeled as animal P1 and animal P2, based on their respective observation periods of 30 days and 90 days, provided valuable insights into the long-term performance and biocompatibility of the LAAC device. The implantation procedure, facilitated by a 14Fr sheath delivery system and continuous fluoroscopic monitoring, ensured precision and accuracy in device placement. Histopathological examination revealed the formation of an epithelial cell layer around the LAAC, confirming the device\'s biocompatibility at both 30 and 90 days post-implantation. The examination of basic functions, including the nitinol alloy wire LAA closure devices, through necropsy demonstrated its integrity. Importantly, scheduled transthoracic echocardiographic examinations at follow-up time points did not reveal any significant pericardial effusion, device-associated mitral valve dysfunction, or pulmonary venous obstruction. Notably, there were no instances of device immobilization or device failure throughout the follow-up period. While both devices displayed time-dependent neo-intima development at 30 and 90 days, this development was found to be entirely endothelialized. Collectively, the novel design and geometry of the aforementioned LAAC device have been thoroughly found to be strong and relevant for its ability to obstruct clot entry into carotid arteries of pigs. This positive outcomes observed with the animal trials established a strong basis for future clinical investigations for reducing the risk of stroke in patients with atrial fibrillation (AF).
References
[1] Kirchhof, P. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37, 2893–2962. https ://doi.org/10.1093/eurhe artj/ehw21 0 (2016). [2] Connolly, S. J. et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151. https ://doi.org/10.1056/NEJMo a0905 561 (2009). [3] Granger, C. B. et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 365, 981–992. https ://doi.org/10.1056/NEJMo a1107 039 (2011). [4] Blackshear, J. L. & Odell, J. A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 61, 755–759. https ://doi.org/10.1016/0003-4975(95)00887 -X (1996). [5] Holmes, D. R. Jr. et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 64, 1–12. https ://doi.org/10.1016/j.jacc.2014.04.029 (2014). [6] Reddy, V. Y. et al. 5-Year outcomes after left atrial appendage closure: From the PREVAIL and PROTECT AF trials. J. Am. Coll. Cardiol. 70, 2964–2975. https ://doi.org/10.1016/j.jacc.2017.10.021 (2017). [7] Glikson, M. et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion: An update. Euro-Intervention https ://doi.org/10.4244/EIJY1 9M08_01 (2019). [8] Landmesser, U. et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: One-year follow-up from the prospective global Amulet observational registry. EuroIntervention 14, e590–e597. https ://doi.org/10.4244/EIJ-D-18-00344 (2018). [9] 14. Boersma, L. V. et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm 14, 1302–1308. https ://doi.org/10.1016/j.hrthm .2017.05.038 (2017). [10] Bellmann, B. et al. Left atrial appendage closure with the new Occlutech(R) device: First in man experience and neurological outcome. J. Cardiovasc. Electrophysiol. 28, 315–320. https://doi.org/10.1111/jce.13141 (2017). [11] Park, J. W. et al. Percutaneous left atrial appendage closure with a novel self-modelizing device: A pre-clinical feasibility study. Int.J. Cardiol. 177, 957–963. https ://doi.org/10.1016/j.ijcar d.2014.09.194 (2014). [12] Bass, J. L. Transcatheter occlusion of the left atrial appendage–experimental testing of a new Amplatzer device. Catheter. Cardiovasc. Interv. 76, 181–185. https ://doi.org/10.1002/ccd.22536 (2010). [13] Bellmann, B. et al. Left atrial appendage closure in a patient with left atrial appendage thrombus using a novel fish ball technique.Int. J. Cardiol. 234, 146–149. https ://doi.org/10.1016/j.ijcar d.2016.12.141 (2017). [14] Scislo, P., Wilimski, R., Zbronski, K. & Huczek, Z. Main pulmonary artery perforations after left atrial appendage occluder implantation. EuroIntervention 14, 894–895. https ://doi.org/10.4244/EIJ-D-18-00419 (2018). [15] Kim, J. S. et al. Preclinical assessment of a modified Occlutech left atrial appendage closure device in a canine model. Int. J. Cardiol. 221, 413–418. https ://doi.org/10.1016/j.ijcar d.2016.07.048 (2016). [16] Schwartz, R. S. et al. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc. Interv. 3, 870–877. https ://doi.org/10.1016/j.jcin.2010.04.017 (2010). [17] Lam, Y. Y. et al. Preclinical evaluation of a new left atrial appendage occluder (Lifetech LAmbre device) in a canine model. Int. J.Cardiol. 168, 3996–4001. https ://doi.org/10.1016/j.ijcar d.2013.06.083 (2013).
Copyright
Copyright © 2024 Dr. Pramodkumar Minocha, Dr. Deveshkumar Mahendralal Kothwala, Arpit Pradipkumar Dave, Mahesh Laxman Pawar, Hrishikesh Mahendra Shah , Sanjay Baijnath Chauhan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET59277
Publish Date : 2024-03-21
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

